We identified a patient with a recently described syndrome, monoclonal gammopathy of thrombotic significance (MGTS; Blood. 2023 Apr 6;141(14):1772-1776), and three additional patients with high 4Ts scores who were consistently negative in the PF4-polyanion HIT ELISA (Lifecodes PF4 IgG, Immucor). The MGTS patient, with a monoclonal IgG kappa, had recurrent thrombosis, and transient mild thrombocytopenia (nadir in the low 100s) over a period of years, and was noted to have a HIT ELISA optical density (OD) of 0.117. The three HIT patients experienced thrombocytopenia and thrombosis with ELISA ODs of 0.37, 0.18 and 0.20. All patients were positive in platelet-activation (functional) testing demonstrating PF4 dependence and inhibited with both high concentrations of heparin (100U/mL) and the FcγRIIa blocking monoclonal antibody (mAb) IV.3, consistent with the presence of anti-PF4 antibodies in the patient samples ( data not shown). Because the serologic profile of these patients suggested a novel HIT subtype characterized by negative HIT ELISA results, we undertook a functional platelet assay screen of 500 consecutive HIT ELISA-negative patients tested in our diagnostic laboratory. Research studies were approved by the Institutional Review Board of Mayo Clinic.
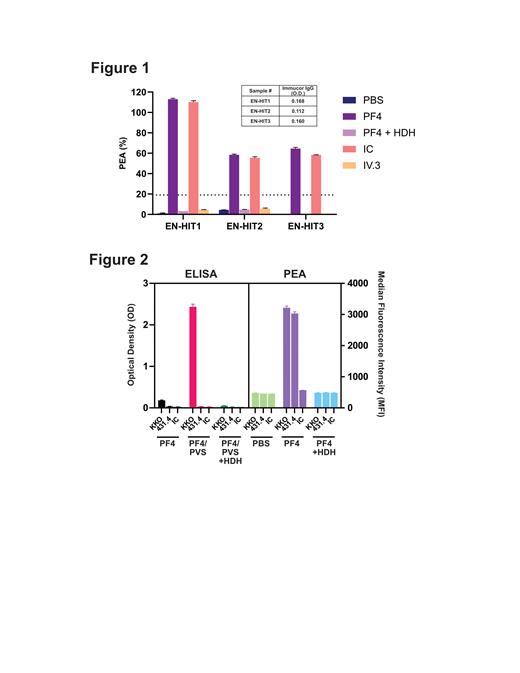
Of the 500 patients tested, 20 (4.0%) stimulated platelet activation in the PF4-dependent P-selectin Expression Assay (PEA ≥19%; data not shown). To exclude non-specific activation that can be induced by HLA antibodies, additional testing was performed to evaluate whether platelet activation was PF4-dependent using buffer or PF4-treated platelets. Three patients demonstrated PF4-dependent activation that was inhibited by high heparin concentrations and mAb IV.3, as would be expected for HIT antibodies (ELISA-negative HIT, EN-HIT1-3; Figure 1). On retrospective review, all three patients thus identified had clinical histories and courses consistent with high clinical probability for HIT.
To develop tools to study this new class of antibodies, monoclonal HIT antibodies were generated in mice after PF4/heparin immunizations and screened using a novel approach that included simultaneous testing of hybridoma supernatants in the PEA and PF4-polyanion ELISA. This strategy was designed to enabled us to identify mAbs that activated platelets (in the PEA), but that did not bind antigenic targets in the HIT ELISA. A novel mAb 431.4 that was developed using this approach, and the well-characterized HIT-like mAb KKO were evaluated for their abilities to both bind PF4-polyanion targets in ELISA, and to activate PF4-treated platelets in the PEA (Figure 2). In significant contrast to KKO, mAb 431.4 did not bind PF4/polyanion complexes, however, like KKO, strongly activated platelets in a PF4-dependent manner. Activation induced by both KKO and mAb 431.4 was inhibited by high heparin concentrations, as would be expected for HIT antibodies. In vivo murine studies using this novel mAb are pending at this time.
In summary, the non-canonical diagnostic profile of HIT ELISA-negative but platelet activation-positive antibodies were found at a relatively high incidence in HIT-suspected patients (0.6%; 3 of 500 ELISA-negative patients). These findings have important diagnostic/management implications and suggest a re-evaluation of ASH's HIT management guidelines as noted in recommendations 2.7 and 2.8 of that document (“In patients with an intermediate (Recommendation 2.7) or high (Recommendation 2.8)-probability 4Ts score and a negative immunoassay, the ASH guideline panel recommends discontinuation of the non-heparin anticoagulant and resumption of heparin, if indicated ”; Blood Adv (2018) 2 (22): 3360-3392).
Figure 1. A subset of ELISA-negative HIT-suspected patients harbor anti-PF4 platelet-activating antibodies. Three ELISA-negative (EN) HIT patient samples from a screen of 500 samples demonstrate PF4-dependent platelet activation and inhibition with high heparin concentration and mAb IV.3 incubation. PBS, phosphate buffered saline; IC, isotype control; HDH- high dose heparin (100U/mL).
Figure 2. Murine mAb 431.4 serologically mimics pathogenic ELISA-negative HIT/MGTS antibodies. ELISA and PEA testing was performed using the “HIT-like” mAb KKO, novel mAb 431.4, or an isotype control (IC) antibody. PVS-polyvinyl sulfonate (polyanion used in the Immucor IgG ELISA assay).
Disclosures
Jones:Retham Technologies: Current equity holder in private company, Patents & Royalties; Versiti Inc.: Patents & Royalties. Murray:Eastman Kodak: Patents & Royalties. Pruthi:HEMA biologics: Consultancy, Honoraria; Instrumentation Laboratories (Werfen): Consultancy, Honoraria; CSL Behring: Consultancy, Honoraria; Genentech Inc.: Consultancy, Honoraria; Bayer Healthcare AG: Consultancy, Honoraria. Pabmanabhan:Retham Technologies: Current equity holder in private company, Patents & Royalties; Veralox Therapeutics: Membership on an entity's Board of Directors or advisory committees; Versiti Inc: Patents & Royalties; Mayo Clinic: Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal